In recent years, monoclonal antibody (mAb) drugs have rapidly developed into a clinically important drug class for a wide range of diseases, including cancer, immunological disorders, infection, and neurological diseases. However, there are still many patients who develop resistance to mAbs. The current challenge in the field of cancer targeted therapy is to develop new drugs with enhanced efficacies to provide effective treatments for those patients develop resistance. Immune-mediated killing mechanism plays a crucial role in the mAb targeted therapy of cancers. In the immune-mediated killing, the Fc portion of antibodies can recruit immune effector cells (e.g., NK cells, macrophages, and dendritic cells) through Fc receptors, allowing for the activation of effector functions that can then cause the lysis of tumor cells. Biological responses induced via the Fc portions of antibodies are powerful and unusual, which can be exploited via the engineering of therapeutic monoclonal antibodies to improve their activity in vivo.
In Fc receptor-mediated cellular cytotoxicity activities, macrophage phagocytosis plays an important role. Traditional research about macrophage phagocytosis was based on optical microscopy, which cannot reveal detailed information because of the 200-nm-resolution limit. The advent of atomic force microscopy (AFM) offers an excellent analytical instrument for quantitatively investigating the biological processes at single-cell and single-molecule levels under native conditions.
Researchers from Shenyang Institute of Automation (SIA), Chinese Academy of Sciences, combined AFM and fluorescence microscopy to visualize and quantify the detailed changes in cell morphology and mechanical properties during the process of Fc receptor-mediated macrophage phagocytosis against cancer cells.
After coincubating the cancer cells and macrophages for 3 hours, the cells were chemically fixed and then imaged by AFM under the guidance of fluorescence microscopy. First, the optical bright field images and fluorescent images were obtained. Then AFM images were obtained. Fig.1 shows the nanoscale cellular morphological changes during macrophage phagocytosis against cancer cells revealed by AFM imaging. Fig.1 A-F shows the cellular morphologies at the initial contact step between a cancer cell and macrophage. Fig.1 G-L shows the cellular morphologies at the beginning of engulfing. Fig.1 M-R shows the cellular morphologies at the step in which the macrophage has engulfed some proportion of the cancer cell.
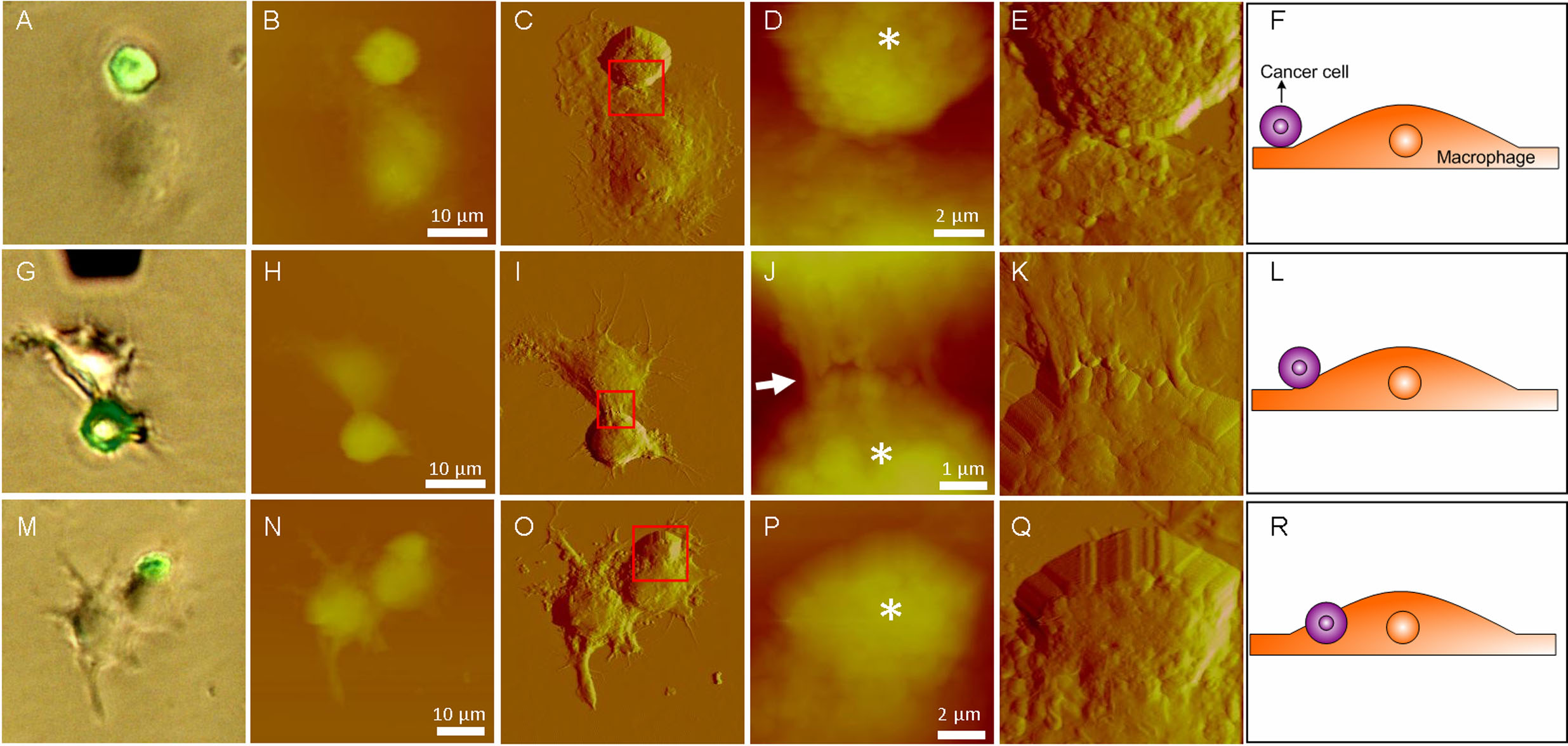
Fig. 1 AFM images reveal the changes in cellular ultramicrostructures during the different steps of macrophage phagocytosis against cancer cells. (A, G, M) Overlay images of optical images and fluorescent images. Cancer cells exhibit green fluorescence. (A−F) Initial contact stage between a cancer cell and macrophage. (G−L) Beginning of engulfing. (M−R) Some proportion of the cancer cell has been engulfed. AFM (B, H, N) height images and (C, I, O) deflection images on a large scale. AFM (D, J, P) height images and (E, K, Q) deflection images on a small scale denoted by the red squares. (D, J, P) Asterisks indicate the cancer cells. (F, L, R) Schematic diagrams of phagocytosis. (Image provided by LI Mi, et.al)
From traditional optical images, researchers cannot see the detailed situations between macrophages and cancer cells. In contrast, the AFM images clearly show the detailed situations at different stages of phagocytosis (initial contact, engulf beginning, some portions had been engulfed), providing novel information that can help researchers to better understand cell biology . Fig.2 shows the changes of cellular mechanical properties during the phagocytosis. Fig. 2A is the Young’s modulus of macrophages with and without cancer cells, clearly showing that macrophages became stiffer after engulfing the cancer cells. While the control experiments (Fig. 2B) shows that the Young’s modulus of macrophages kept stable if the phagocytosis was not stimulated, demonstrating that the changes of cellular mechanical properties were caused by the phagocytosis. These results will be particularly useful for the researchers to understand macrophage phagocytosis against cancer cells.
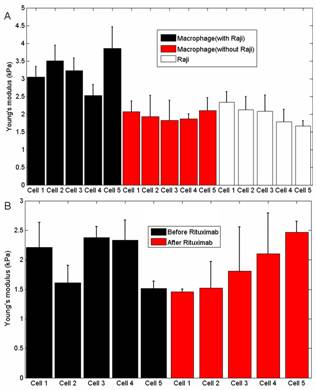
Fig.2 Measuring the mechanical properties of macrophages during phagocytosis. (A) Young’s modulus of macrophages (with and without cancer cells) and cancer cells. (B) Young’s modulus of macrophages before and after the stimulation of rituximab. (Image provided by LI Mi, et.al)
This work has demonstrated that the use of integrated AFM fluorescence microscopy to visualize and quantify the nanoscale cellular properties (ultramicrostructure, surface roughness, and mechanical properties) successfully during antibody-dependent Fc receptor-mediated macrophage phagocytosis against cancer cells. In future studies, the researchers plan to visualize the real-time cellular morphological changes during the process of phagocytosis on living cells with time-lapse AFM.
This work was published on Langmuir, vol. 30, pp.1609-1621, 2014. It was partly supported by the National Natural Science Foundation of China (61175103), and the CAS FEA International Partnership Program for Creative Research Teams.
CONTACT:
Mi Li
Shenyang Institute of Automation, Chinese Academy of Sciences
E-mail: limi@sia.cn