Ion channels, the transmembrane proteins, can be classified into three main types: voltage-gated, ligand-gated, and mechanosensitive (MS), according to their gating nature. These ion channels regulate important functions of living cells. For example, MS ion channels response to the phospholipid bilayer membrane deformation, which plays an essential role in turgor regulation and osmotic pressure balance. Malfunction of MS channels may cause disorder of many physiological processes, including touch, pain, proprioception sensation, hearing, blood pressure adjustment, body fluid balance. Furthermore, some abnormalities may contribute to cardiac arrhythmia and even worse disorders. However, studies on these mechanisms of MS channels are limited due to lack of effective instrument for study MS channels in situ.
To solve this problem, researchers from Shenyang Institute of Automation (SIA), Chinese Academy of Sciences developed a mechanostimulated patch-clamp system in Fig.1 through integrating a custom-designed planar patch-clamp with a robot-assisted AFM on an inverted fluorescence microscope recently. In this system, the mechanostimulated patch-clamp system can achieve automatic patching, exchanging intracellular solution, nano-scale manipulation, accurate positioning, diversified stimuli, friendly human-machine interaction, and simultaneous signal recording. Since operators can obtain electrical, mechanical and fluorescent signals simultaneously from the mechanostimulated patch-clamp system, it can be a powerful tool in study of MS ion channels, which requires versatile stimuli under precise control.

Fig. 1. The schematic diagram of the mechanostimulated patch-clamp system. (a) AFM cantilever as the end effector of the system; (b) The recording system of the planar patch-clamp module; (c) The inverted microscope platform. (Image provided by ZHANG Changlin et.al.)
As verification of the system functions, the researchers tested the planar patch clamp by performing whole-cell recording on HEK293 cell which over expresses Kv1.1 potassium ion channel and the results were showed in Fig.2. The K-channel currents shown in Fig.2(a) and Fig.2(b) were elicited using 600 ms voltage steps from a holding potential of -80 mV to -90 mV up to +80 mV (10 mV increments, sweep interval 2 s) followed by a step back to the holding potential shown in Fig.2(c). The gate voltage is about -10 mV. This result is consistent with other studies, which proves the effectiveness of the custom designed planar patch clamp.
Then, the researchers tested the whole system on Neuro2A cell, which had recently been known expressing some MS ion channels (Piezo1 and Piezo2). In this test, the AFM tip was held in the extracellular solution for 10 s and then moved down about 1-2 μm onto the capturing cell with a bended deformation of the cantilever about 0.3-0.5 V. After that, the tip was held on the cell for 2 s and then moved back to original position. This process was repeated in the same place and the researchers observed the relevant cell current. The result was shown as Fig. 3. Finally, the researchers verified the ability of the system to exchange intracellular solution, which have significant potential in the study of ion channels. After performing whole-cell recording, the intracellular solution was exchanged by propidium iodide (PI, Sigma Aldrich) solution (0.05 mg/ml concentration prepared with intracellular solution) through the microfluidic channel in the chamber. Only the captured cell turning red under the green fluorescence were shown as Fig. 4(d), which was clear evidence that the intracellular solution can be successfully exchanged. This experiment verifies the ability of the designed system to exchange intracellular solution during patch-clamp recording.

Fig. 2. Recording of voltage-gated ion channel Kv1.1 on HEK293 cell. (a) Whole-cell current recording without adding TEA blocker; (b) Whole-cell current recording 30 s after adding TEA blocker; (c) The relative voltage stimulate protocol. (Image provided by ZHANG Changlin et.al.)
(a)
(b)
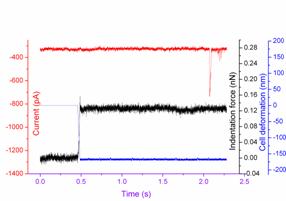
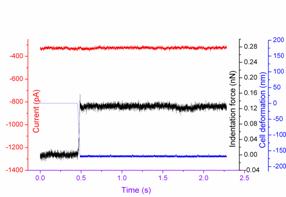
Fig.3. Current responses to the AFM tip indentation. The black line represents the action of the AFM tip (force); the blue line represents the cells deformation; while the red line represents the subsequent mechanical ion channel current (a) without channel inhibitor GdCl3 (b) with channel inhibitor GdCl3. (Image provided by ZHANG Changlin et.al.)

Fig. 4. Process of exchanging intracellular solution: (a) The clean planar chip setup before adding living cell. (b) Capture one cell to perform whole-cell recording. (c) Exchange intracellular solution with PI solution. (d) Expose to green fluorescence after wash the PI solution away with intracellular solution. (Image provided by ZHANG Changlin et.al.
This work was published on IEEE/ASME Transactions on Mechatronics, Issue 99. It was partly supported by the National Natural Science Foundation of China (60904095, 61175103), National 863 Project of China (No. 2009AA03Z316), the CAS/SAFEA International Partnership Program for Creative Research Teams, and the National Science Foundation (CNS 1035563).
CONTACT:
Zhang Changlin
Shenyang Institute of Automation, Chinese Academy of Sciences
E-mail: zhangchanglin@sia.cn